WHAT IS HEAT
INTRODUCTION:
Heat is a form of energy in transit, which excites us in the sensation of warmth. It is a form of enegy which floes from a body at a higher temperature to a body at a lower temperature. In physics the term heat refers to energy in transit from one body to the other because of the temperature difference, never to the amount of energy contained within a system.
FACTORS ON WHICH HEAT REQUIRED TO INCREASE TEMPERATURE
Q is the amount of heat which is needed to increase the temperature of a body having mass M that is proportional to M ( Q ∞ M). The factors on which heat required to raise the temperature of a body depends upon
i)Mass of the body
ii)The rise in temperature
iii)The nature of the substance of which the body is made
UNIT OF MEASURING HEAT ENERGY:
Unit for measurement of heat enegy are as follows:
CALORIE: The calorie ( cal) is defined as the amount of heat required to raise the temperature of one gram of water through 1°C (exactly from 14.5°C to 15.5°C).
KILOCALORIE: The kilocalorie (kcal) is defined as the amount of heat required to raise the temperature of one kilogram of water through 1°C (from 14.5°C to 15.5°C)
1 kilocalorie = 1000 calorie.
joule(J) : In SI system, the unit of heat is joule.
1 calorie = 4.186 joule
CONDUCTORS OF HEAT :
The transfer of heat through a metal can be explained by atomic vibrations. We can hold a piece of asbestos in a flame for long time but a metal piece is impossible to hold in flame by hand. In general, metals are good conductors of heat e.g. Aluminium, Iron, Copper etc. These are good conductors because they contain large number of free electrons that are relatively free to move through the metal and transport energy one region to the other . In this way, in a good conductor, heat conduction takes place via the vibration of atoms through the motion of free electrons.
Some materials are bad conductors of heat like Asbestos, cork, Paper, Fibres and Glass. Gases are also poor conductors of heat because of their dilute nature. That is why cork fibres , plastics are used as handle of utensils so that we can hold them safely.
GOOD CONDUCTORS: These are the substances which readily conduct heat.
BAD CONDUCTORS: These are the substances which do not conduct it.
USES OF CONDUCTORS : These are many uses of good and bad conductors of heat . Some of them are
| TYPE OF CONDUCTORS | EXAMPLE | SPECIALITY/CHARACTERS | DIAGRAM |
| GOOD CONDUCTORS | Copper vessel | Base of some cooking vessel is made of copper which allows heat to flow quickly from the flame to the cooking material. |  |
| GOOD CONDUCTOR | Copper kettle | Tea-kettles have copper bottom to ensure greater conductivity of heat. |  |
| GOOD CONDUCTOR | Hot water metal radiator | It is used for heating up rooms in western countries so that heat energy from the hot water inside is conducted quickly to the outside of the radiator. | 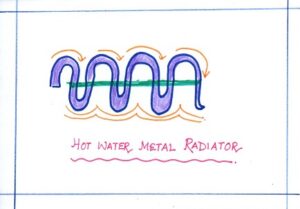 |
| GOOD CONDUCTOR | Steel boilers in industry | It is used to conduct heat quickly from the source of heat to the liquid being boiled. | 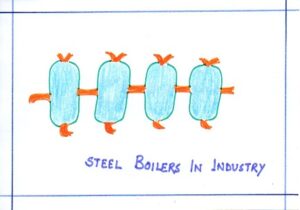 |
| BAD CONDUCTOR | Plastic | Handle of hot vessels like sauce pan, kettle are fitted with these material to prevent hotness. |  |
| BAD CONDUCTOR | Gloves | Made of silicon, rubber so that heat can be prevented. | 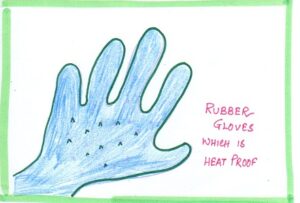 |
| BAD CONDUCTOR | Table mat | These are made of non-conducting material like wool, cloth, wood so that hot plates do not damage the table surface. | 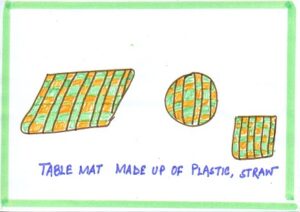 |
| BAD CONDUCTOR | Oven | It has double wall and lining of cotton wool or glass wool to minimize loss of heat to surrounding. |  |
| BAD CONDUCTOR | Woollen sweater | Air is a bad conductor and it is trapped within wollen garment to make us warm in winter. |  |
EFFECTS OF HEAT: ( WHAT IS HEAT)
When a body is heated it undergoes a number of changes. Some of these changes are:
- CHANGE IN TEMPERATURE: When a matter (solid, liquid, or gas) is supplied with heat energy, its temperature rises. Conversely , if a hot matter gives out heat energy, its temperature falls.
- CHANGE IN DIMENSION: when a body (solid, liquid or gas) is supplied with heat energy, its dimension i.e. length, area or volume is changed.
- CHANGE IN STATE: This supplied to a body can also cause the change in its state. When a solid is heated, initially its temperature rises, then it becomes stable. After that solid turns into liquid and liquid by further heating turns into gaseous state. Conversely, when a gas loses heat energy, it changes liquid state and then to solid state by condensation.
- CHEMICAL CHANGE: when the reactants are heated, large number of chemical reactions take place e.g. Potassium chlorate( KClO3) on heating decomposes to form Potassium chloride( KCl) and Oxygen(O2) gas.
- EFFECT ON LIVING BEING: All mammals functions effectively at some fixed body temperature. The body temperature of human being is 37°C or 98.6°F.If the surrounding temperature is more or lower than the body ( in too hot or too cold condition) , the functions of body slow down. Similarly, plant and other animals sometimes may die in very cold or hot climate. That is why when we boil milk or water, the bacteria present inside the liquid is killed.
EXPANSION DUE TO HEATING : Most of the materials increases their dimension by increase of temperature. Rising temperature make the liquid expand in a liquid-in tube thermometer and bend bimetallic strips. The bridge deck contains special type of joints which helps to support expansion on heating. A completely filled and tightly capped bottle of water cracks when it is heated, we can loosen a metal jar lid by running hot water over it. These are all examples of thermal expansion. There are three types of thermal expansion:
- Linear expansion
- Superficial expansion
- Cubical expansion
LINEAR EXPANSION:
In this expansion when a solid is heated there is a change in this length alone. In linear expansion, when the temperature of a body is raised its length increases. Inncrease in length depends upon:
- Nature of material
- Original length og the body
- The increase in temperature
SUPERFICIAL EXPANSION: ( WHAT IS HEAT)
In this expansion, when a solid is heated there is a change in its surface area alone. In this type of expansion, when the temperature of a body is raised, its surface area increases. The increase in the surface area depends upon:
- Nature of material
- Original surface area of the body
- The increase in temperature
CUBICAL EXPANSION: In this expansion when a solid is heated there is a change in its volume. In this expansion, when the temperature of a body is raised, its volume increases. The increase in volume depends upon:
- Nature of material
- Original volume of the body
- The incrase in temperature
There are various applications of thermal expansion, like iron tyre of horse cart, riveting, overlapping of railway track, expansion of joints in metal pipe, roller under bridge etc. which will be explained later.