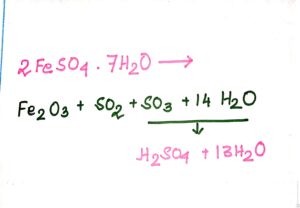INORGANIC ACID
INTRODUCTION IN INORGANIC ACID
Three main inorganic acid or mineral acids are HYDROCHORIC ACID, NITRIC ACIDS, SULPHURIC ACID. Acids were derived from the latin word meaning sour , since they are associated with sour taste of various fruits.
DISCOVERY OF INORGANIC ACID
HYDROCHLORIC ACID |
NITRIC ACID |
SULPHURIC ACID |
| · A solution of hydrogen chloride gas was named as muriatic acid.
· In 1648 Glauber prepared HCl gas from rock salt and conc.H2SO4. · In 1772 Joseph Priestly obtained HCl in pure form and named its solution marine acid. · Later it was named muriatic acid by Lavoisier. · In 1810 Davy gave the name Hydrogen Chloride. |
· Initially it was known as aqua fortis in 8th century by Alchemists means strong water.
· Aqua fortis means it has result of its corrosive action on many metals.· In 1658 Glauber prepared HNO3 by distilling nitre [KNO3] with conc.sulphuric adid. · In 1784 Cavendish determined the composition of Nitric acid . |
· Initially it was called oil of vitriol.
· Initially it was prepared by distilling green vitriol [FeSO4.7H2O]. |
OCCURRENCE OF INORGANIC ACID
| HYDROCLORIC ACID | NITRIC ACID | SULPHURIC ACID |
R
|
· It occurs as free state during lightning discharge by following way:
N2 + O2 lightning discharge à 2NO2NO + O2 2NO2 4NO2+ 2H2O => O2 + 4HNO3 [NITRIC ACID –acid rain] 2HNO3+ CaCO3 Ca(NO3)2 + H2O + CO2. Soluble nitrates in soil. · In combined state as salts in minerals e.g. chile salt peter (NaNO3); bengal salt petre or nitre (KNO3). |
· It occurs as free state in hot spring.
· It is also formed near sulphide beds e.g. by hydrolysis of iron pyrites. · In the combined form as it occurs as salt in minerals like metallic sulphate. |
LABORATORY PROCESS OF INORGANIC ACID PREPARATION
| HYDROCHLORIC ACID | NITRIC ACID | SULPHURIC ACID |
| · By synthesis or direct combination of
H2+ Cl2à2HCL (g) (explosive in direct sunlight ; negligible in the dark) moisture acts as a catalyst. · Metallic chloride + H2SO4(conc) <200°c à Metallic bisulphate + HCl(g) |
· Nitre + conc. H2SO4 <200°C HNO3 (vapour) + Potassium bisulphate.
· Chile salt petre + H2SO4 <200°c Sodium bisulphate + HNO3 (vapour) |
· S + HNO3 (conc) à 6NO2 + 2H2O + H2SO4
(conversion of HNO3 to H2SO4)· Cl2 + SO2 + 2H2O à 2HCl + H2SO4 · SO3 + H2O àH2SO4 · SO2Cl2+ 2H2O à H2SO4+ HCl |
EXPLANATION OF EACH LABORATORY PROCESS IN ORGANIC ACID
1)HYDROGEN CHLORIDE
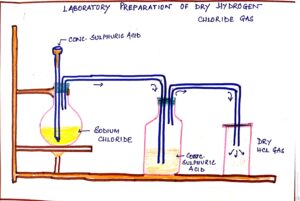
FIG#1 Simplified diagram of laboratory preparation of Hydrogen chloride.
REACTANT |
PRODUCT |
EQUATION |
FORMATION |
PURIFICATION |
COLLECTION |
| Rock salt or sodium chloride (NaCl) | Sulphuric acid (conc) | NaCl + H2SO4 <200°c à NaHSO4 + HCl (g) | Gas is obtained in the round bottom flask, then passed to washer ottle for drying. | Washer bottle contains drying agent conc. H2SO4 which absorbs moisture. | Dry HCl gas is collected by the upward displacement of air as it is heavier than air. |
PRECAUTIONS :
- Reactions mixture is initially heated to control the evolution of HCl gas which is an inorganic acid.
- Lower end of thistle funnel should dip below the acid in flask to obtain HCl gas properly otherwise it will escape out from the thistle funnel.
- Hydrogen chloride gas is extremely soluble in water .
- This gas is passed in water to make hydrochloric acid but if a delivery tube through which HCl gas is passed is directly immersed in water then the rate of absorption of HCl gas is high and partial vacuum is created.
- This phenomenon causes water to be pushed into delivery tube and damages the apparatus. This is called BACK SUCTION.
To prevent this problem special funnel arrangement is used for avoiding back suction. This arrangement
- Prevents or minimizes back suction.
- Provides a large surface area for absorption of hydrogen chloride gas.
- By this process pressure is equalized in both outer and inner side
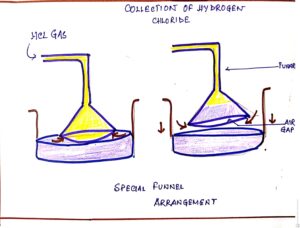
FIG#2 Special funnel arrangement
Hence hydrochloric acid which is an inorganic acid is not prepared in the laboratory by passing hydrogen chloride gas directly through water, but prepared using special funnel arrangement.
2) NITRIC ACID
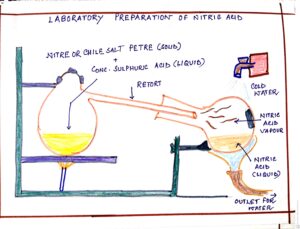
FIG#3 Laboratory preparation of Nitric acid
REACTANT |
PRODUCT |
EQUATION |
FORMATION |
COLLECTION |
| i) Nitre and conc. Sulphuric acid
ii) Chile salt petre + conc. Sulphuric acid. – |
Potassium bi sulphate and Nitric acid (vapour).
Sodium bi sulphate + Nitric acid (vap) |
KNO3 + H2SO4(CONC) <200°c à KHSO4 + HNO3
NaNO3+ H2SO4 <200°cà NaHSO4 + HNO3 |
Volatile nitric acid vapour is collected in the receiver which is cooled from outside with cold water.
Volatile nitric acid vapour is collected in the receiver which is cooled from outside with cold water. |
Conc. Nitric acid vapours are condensed and collected in the water-cooled receiver.
Concentrated Nitric acid vapour is condensed and collected in the water-cooled receiver.
|
-
PRECAUTIONS IN PREPARATION OF INORGANIC ACID
Complete apparatus is made up of glass as vapour of HNO3 is highly corrosive .
- HCl cannot be used in the place of conc.H2SO4 as conc.H2SO4 is a strong non-volatile acid and produce HNO3 is a volatile acid; HCl is more volatile than HNO3.
- Reaction temperature must be less than 200°c as high temperature above 200°c may damage the apparatus, and also decomposes HNO3.
- More than 200°c may form hard residual Na2SO4
(2 NaNO3 + H2SO4 [conc.] >200°cà Na2SO4 + 2HNO3)
- Sodium sulphate is a poor conductor of heat and sticks to the glass, which cannot be removed from the apparat us easily.
INDUSTRIAL METHOD OF NITRIC ACID AS INORGANIC ACID BY OSTWALD PROCESS
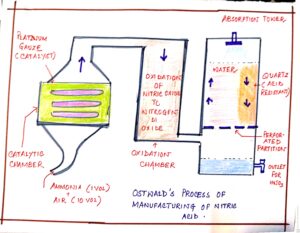
FIG#4 Ostwald’s process of nitric acid preparation
CHAMBER |
REACTANT |
CATALYST |
TEMPERATURE |
NATURE OF REACTION |
EQUATION |
| CATALYTIC CHAMBER | PURE DRY AMMONIA + DRY AIR | Platinum gauze | 700°c – 800°c | exothermic | 4NH3+ 5O2 à 4NO + 6H20 |
| OXIDATION CHAMBER | NITRIC OXIDE + OXYGEN | Not required | 50°c | oxidation | 2NO + O2 à 2NH3 |
| ABSORPTION TOWER | NITROGEN DI OXIDE + WATER + OXYGEN | Not required | Normal temperature | absorption | 4NO2 + 2H2O + O2 à 4NH3 |
FLOW CHART
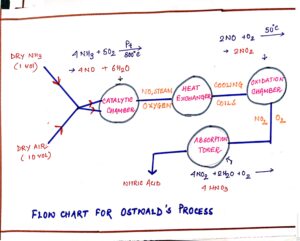
FIG#5 Simplified fow chart of Ostwald’s process
PRECAUTIONS: IN PREPARATION OF INORGANIC ACID
- In catalytic chamber 1 volume of pure dry ammonia and 10 volumes of dry air are used. Higher ratio of air is used as air is required in all the three reactions i.e. the reactions in the catalytic, oxidation and absorption tower.
- In catalytic chamber the catalyst platinum gauze is initially heated – as the reaction is exothermic in nature, the electrical heating is done initially only.
- The gases leaving the catalytic chamber are passed through cooling coils before entering the oxidation chamber to lower temperature since they facilitate easy oxidation of nitric oxide to nitrogen di oxide and they minimize the chances of any decomposition of nitrogen di oxide which may take place at higher temperature.
- The gases leaving the oxidation chamber enter the absorption tower which is packed with quartz in layers as quartz is acid resistant and quartz packing in layers slow down the movement of the gaseous NO2 entering from below and initiates better salvation of nitrogen di oxide in water.
- Dilute nitric acid is concentrated by distillation or boiling until a constant boiling mixture is obtained.
INDUSTRIAL METHOD OF PREPARATION OF SULPHURIC ACID OR INORGANIC ACID BY CONTACT PROCESS
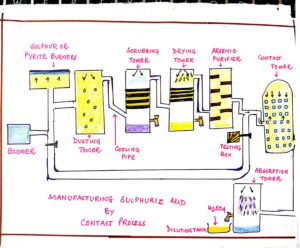
FIG#6 Manufacturing process of Sulphuric acid by contact process
| PARTS OF UNIT | FUNCTION | RELATED REACTION WITH DIAGRAM | |||||||||||||
| A: PRODUCTION of SO2
i) BLOWER ii) SULPHUR OR PYRITE BURNER. |
i) For passage of purified air or oxygen.
ii) For production of sulphur di oxide by burning sulphur or iron pyrites. |
S + O2 => SO2
4FeS2 + 11O2 à 2Fe2O3 + 8 SO2 |
|||||||||||||
B: PURIFICATION UNIT (FOR PURIFICATION OF GASEOUS MIXTURE OF SO2 AND O2)
|
|
||||||||||||||
C: CATALYTIC OXIDATION (CONTACT TOWER IN INORGANIC ACID |
For catalytic oxidation of SO2 to SO3 by passage of sulphur di oxide and oxygen through iron tower packed with vanadium pentoxide catalyst initially heated to 450°c. | 2SO2 + O2 ßà(V2O5)
2SO3 + HEAT CATALYST: Vanadium pentoxide or Platinum TEMPERATURE: 450°c – 500°c PRESSURE: 1 or 2 atm. CONVERSION RATIO: 98% SO2 converted to SO3. |
|||||||||||||
|
|
|
|||||||||||||
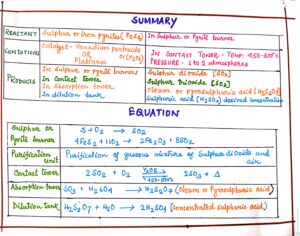
FIG#7 Summary of sulphuric acid manufacturing
PRECAUTION IN PREPARATION OF INORGANIC ACID
During burning of sulphur or iron pyrites oxygen is preferred to purified air since heat energy is wasted in heating the unreactive nitrogen components of the air.
In purification unit the impurities of pyrite dust and arsenious oxide are removed to prevent deactivation of catalyst and reduction of efficiency. ( the fresh fruits and vegetables conducts electricity to some extent. explain why)
During contact tower catalytic oxidation takes place in presence of catalyst vanadium pentoxide (V2O5); this is preferred to platinized asbestos as a catalyst since it is comparatively cheaper and led easily poisoned.
Catalyst is initially heated as catalytic oxidation which is an exothermic reaction and the heat produced maintains the temperature at 450°c – 500°c.
The optimum pressure is about 1 to 2 atmosphere as high pressure favours a higher yield of sulphur tri-oxide.
In the absorption tower the SO3 vapours are absorbed in conc. INORGANIC ACID and not in water. If SO3 is directly absorbed in water , then the reaction will be highly exothermic resulting in the production of dense fog of sulphuric acid particles which donot condense easily.