CLASSIFICATION OF MATTER
The material of which the universe is composed is called matter. Matter may be defined as something that occupies space, possesses mass and offers resisance to any stress or force applied on it. Air, water, solid rock, soil etc. are some typical forms of matter. Heat, light, electricity sound etc. are not considered matter because they donot posses mass nor do they occupy space. There are two types of matter LIVING and NONLIVING. In the case of non-living matter there are three states of matter.
STATES OF MATTER ( CLASSIFICATION OF MATTER)
According to the physical state, there are three states of matter: SOLID, LIQUID, GAS.
SOLID: In solids, the particles are closely packed and bound to each other firmly.
As a result, the particles in solids cannot move freely.
LIQUID: In liquids, the particles are somewhat loosely packed.
So these particles can move around easily within the liquid but are not able to escape.
GAS: In gases, the force of attraction between the particles is negligible.
The particles in gases are free and can move with high speed in any direction. ( CLASSIFICATION OF MATTER)
COMPARATIVE STUDY OF SOLID, LIQUID, GAS
| CHARACTER | SOLID | LIQUID | GAS |
| 1)Volume | Definite | Definite | Not definite |
| 2)Shape | Definite shape | Can take shape of container | No shape at all |
| 3)Rigidity | Very regid | Less rigid | No rigidity found |
| 4)Molecular arrangement | Closely packed | Loosely packed | Very loosely packed |
| 5)Inter-molecular space | Very less space in between the molecules | Larger intermolecular space than solid | There are a lot of spaces in between the particles. |
| 6)Intermolecular force | Maximum force | The force is lesser than solid | Minimum force |
| 7) Fluidity | Cannot flow at all ( WHAT’S THE MATTER) | Can flow | Can flow rapidly |
| 8)Kinetic energy | Very little KE in between the particles | More kinetic energy than solid | Large kinetic energy |
| 9)Example |  |
 |
 |
KINETIC MOLECULAR THEORY OF MATTER ( CLASSIFICATION OF MATTER)
The description of matter in terms of the motion of molecules or atoms or ions in it is called the kinetic molecular theory of matter. The main postulates of the kinetic molecular theory of matter are:
- All substances ( solid, liquid and gases) consist of particle (atoms, ions or molecules)
- These particles are in a state of constant motion and therefore, posses kinetic energy.
In SOLID, the particles are closely packed, and bound to each other firmly.
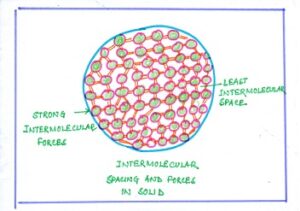
Fig#1 intermolecular space of solid
As a result, the molecules of solid materials cannot move freely. However, the particles can vibrate about their mean position.
In LIQUID, the particles are somewhat loosely packed, so these particles can move around easily within the liquid but are not able to escape. Therefore, the particles in any liquid have more kinetic energy than in a solid state. ( WHAT’S THE MATTER)
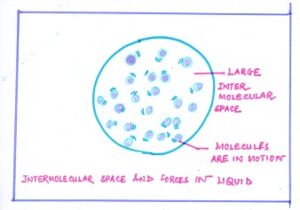
Fig#2 intermolecular space in liquid
In GASES the force of attraction between the particles is negligible. The particles in gases are free and can move with considerable speeds in any direction.
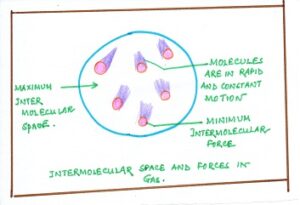
Fig#3 intermolecular space in gases
Thus, according to kinetic molecular theory of matter, the kinetic energy of the molecules of a substance is lowest in the solid state and very high in the gaseous state.
LAW OF CONSERVATION OF MASS
A French scientific expert, Antoine Lavoisier, who is known as the godfather of science changed the type of science from a subjective to a quantitave science. He proved that the total mass of the products in a chemical reaction is equal to the total mass of the reactants. He named this as the law of conservation of mass, stated as follow. ( CLASSIFICATION OF MATTER)
This law states that matter can neither be created nor destroyed in a chemical reaction; it is universal.
INTERCONVERSION OF STATES OF MATTER
It is the phenomenon of change of matter from one physical state to another and back to its original state by a change in the conditions of temperature and pressure. ( CLASSIFICATION OF MATTER)
BY CHANGE IN TEMPERATURE: By increasing temperature, a solid can be changed into a liquid and a liquid can be changed into a gas. This is because on increasing temperature, the kinetic energy of the molecules increases and they bcan move freely which results in the increase of spaces resulting in a change of state. The reverse changes occur on cooling.
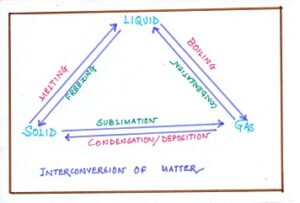
Fig#4 interconversion of matter
Matter can be converted from one state to another by a simple process like freezing, boiling, melting, etc. For example, water can be converted to gaseous form by boiling and ice can be converted to water by melting. When matter is converted from one state to the other, it is called a change of state. Let us understand the various processes that taken place during change of state. ( Matter)
There are various distinct changes of phases which hapeens to different substances at different temperature. These changes are:
BOILING—A process in which a substance changes its state from the liquid state to the gaseous state. Boiloing is a fast process in which bubbles are formed and occurs at a definite temperature called the boiling point. It takes place throughout the liquid. It requires a source of energy. (CLASSIFICATION OF MATTER)
EVAPORATION OR VAPORIZATION—A process in which a substance changes its state from the liquid to the gaseous state without boiling. It is slow and natural process occurs at any temperature. In this process liquid changes into gas as particles escape to move around independently without any constraints.
MELTING—A process in which a substance changes from the solid to the liquid. The temperature at which this change happens is called melting point. Each solid has a fixed melting point at normal air pressure. At lower air pressure ( such as on a mountain), the melting point decreases.
FUSION—A process in whicyh solid melts by applying excess heat from outside. When heat is applied, energy is taken by a solid and the particles start vibrating at high speed. Finally, the vibrations become energetic enough to overcome the molecular force of attraction and particles start sliding out their positions to flow about for fusion.
CONDENSATION—It is a process in which a substance changes from a gas to a liquid. Water that is present as a gas in the air cools down and changes into tiny drops of liquid water on the leaves and window. ( Matter)
SUBLIMATION—It is a process in which a substance changes directly from a solid to a gas without going through the liquid phase. It is a physical change in which camphor, naphthalene ball turns into vapour. ( CLASSIFICATION OF MATTER)
DEPOSITION—It is a process in which a substance changes directly from a gas to a solid without going through the liquid phase. It occurs when gas particles become very cold. The formation of frost of ice crystal on the window pane is an example of deposition.
FREEZING—It is a process in which a substance changes from a liquid to a solid. For example, liquid rock lava) errupted from volcano with high temperature when falls on cold earth-surface, it turns back to solid rock by freezing.